
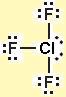
The octet rule refers to each atom’s desire or natural tendency to have 8 electrons on its valence shell by losing, acquiring, or sharing electrons. Similarly, fluorine has seven electrons on the outermost shell thus making the number of valence electrons of fluorine seven. Hence, the total number of valence electrons on chlorine is seven. Chlorine has seven electrons on the outermost shell. They are either transferred or shared (completely or partially) for the bond formation. Only the valence electrons take part in any chemical bond formation. The total number of electrons present on the outermost shell of an atom is called valence electrons. Thus, three fluorine atoms are bonded to a central chlorine atom. It is because Chlorine is less electronegative (3.16) than Fluorine (3.98) and we know the lesser the electronegativity value of an element the more likely it is to behave as a central atom. In this molecule, chlorine acts as the central atom. There is one chlorine atom and three fluorine atoms in a chlorine trifluoride molecule. In a molecule’s Lewis structure, a dot represents a lone pair of electrons and a straight line represents a bonded pair of electrons.Ĭhlorine Trifluoride or Chlorotrifluoride consists of two types of elements, Chlorine, and Fluorine. The Lewis structure only considers a molecule’s valence shell electrons, ignoring the internal shells. The Lewis structure also depicts the total number of lone and bonded pairs of electrons in the atom. Lewis structure, also known as electron dot structure, is a diagrammatic representation of molecule atom bonding. It has a vapor pressure of 1.55 kg/cm 2 at 21☌.The melting and boiling point of Chlorine trifluoride is -76.34☌ and 11.75☌ respectively.It has a molar mass of 92.448 g/mol and a density of 1.77 g/cm 3.It undergoes hydrolysis reaction with water and causes thermal burn in case of exposure.It is highly corrosive in nature and causes severe problems when inhaled.It is colorless, toxic with highly reactive properties.The symptoms of chlorine trifluoride poisoning are seen much later from the time of exposure to it. It is more dangerous because it does not show the effects of poisoning immediately after exposure.
CLF3 MOLECULAR GEOMETRY SKIN
It causes permanent skin burns and ulcers. It is corrosive to the skin, eyes, nose, and throat. It is used as a pyrolysis inhibitor for fluorocarbon polymers.Ĭhlorine trifluoride is an extremely toxic, poisonous, and corrosive gas.It can be used as a fluorinating agent.It is used to clean chemical vapor deposition chambers in the semiconductor industries.

( but it is not yet used due to potential health hazards)
CLF3 MOLECULAR GEOMETRY FREE
It reacts violently with water and gives off hydrogen fluoride (HF), hydrogen chloride (HCl), and free oxygen (O 2). Chlorine trifluoride is an oxidizing and fluorinating agent. It is highly poisonous and corrosive and is an extremely reactive gas. It has an irritating and pungent characteristic smell. Chlorine trifluoride or Chlorotrifluoride (ClF 3) is an interhalogen colorless unpleasant chemical compound with toxic properties.


 0 kommentar(er)
0 kommentar(er)
